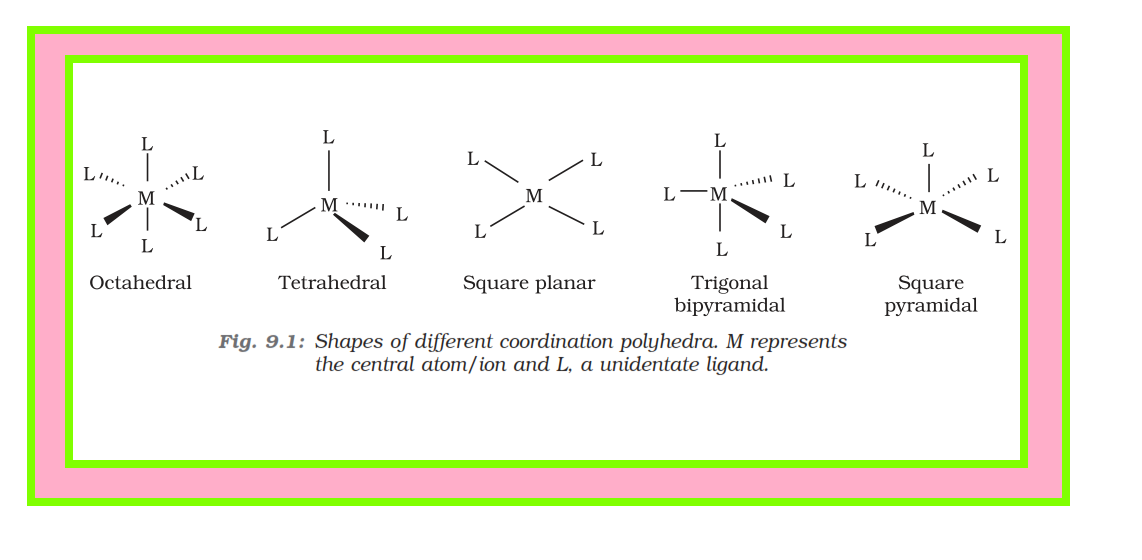
Ligands :
`color{green}("Definition" )` : The ions or molecules bound to the central atom/ion in the coordination entity are called ligands.
● These may be simple ions such as `color{red}(Cl^-)`, small molecules such as `color{red}(H_2O)` or `color{red}(NH_3)`, larger molecules such as `color{red}(H_2NCH_2CH_2NH_2)` or `color{red}(N(CH_2CH_2NH_2)_3)` or even macromolecules, such as proteins.
`color{green}("Types of Ligands based on Denticity ") : `
`color{green}("Denticity ")` : The number of ligating groups in a ligand which can bind to the central metal atom/ion is called the denticity of the ligand.
(i) `color{green}("Unidentate Ligands ")` : When a ligand is bound to a metal ion through a single donor atom, as with `color{red}(Cl^-)`, `color{red}(H_2O)` or `color{red}(NH_3)`, the ligand is said to be unidentate.
(ii) `color{green}("Didentate Ligands ")` : When a ligand can bind through two donor atoms as in `color{red}(H_2NCH_2CH_2NH_2)` (ethane-1,2-diamine) or `color{red}(C_2O_(4)^(2–))` (oxalate), the ligand is said to be didentate.
(iii) `color{green}("Polydentate Ligands ")` When several donor atoms are present in a single ligand as in `color{red}(N(CH_2CH_2NH_2)_3)`, the ligand is said
to be polydentate.
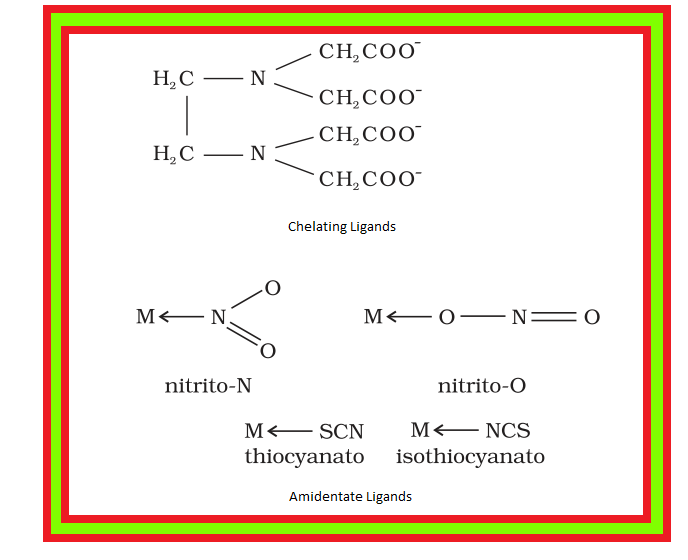
`color{red}("Example ")` : Ethylenediaminetetraacetate ion (`color{red}(EDTA^(4–))`) is an important hexadentate ligand. It can bind through two nitrogen and four oxygen atoms to a central metal ion.
(iv) `color{green}("Chelate Ligands ")` : When a di- or polydentate ligand uses its two or more donor atoms to bind a single metal ion, it is said to be a chelate ligand.
● Complexes formed by chelating ligands are called chelate complexes.
● The complexes tend to be more stable than similar complexes containing unidentate ligands.
(v) `color{green}("Ambidentate Ligands ")` Ligand which can ligate through two different atoms is called ambidentate ligand.
● Examples of such ligands are the `color{red}(NO_(2)^-)` and `color{red}(SCN^-)` ions.
`->` `color{red}(NO_(2)^-)` ion can coordinate either through nitrogen or through oxygen to a central metal atom/ion.
`->` `color{red}(SCN^-)` ion can coordinate through the sulphur or nitrogen atom.
● These may be simple ions such as `color{red}(Cl^-)`, small molecules such as `color{red}(H_2O)` or `color{red}(NH_3)`, larger molecules such as `color{red}(H_2NCH_2CH_2NH_2)` or `color{red}(N(CH_2CH_2NH_2)_3)` or even macromolecules, such as proteins.
`color{green}("Types of Ligands based on Denticity ") : `
`color{green}("Denticity ")` : The number of ligating groups in a ligand which can bind to the central metal atom/ion is called the denticity of the ligand.
(i) `color{green}("Unidentate Ligands ")` : When a ligand is bound to a metal ion through a single donor atom, as with `color{red}(Cl^-)`, `color{red}(H_2O)` or `color{red}(NH_3)`, the ligand is said to be unidentate.
(ii) `color{green}("Didentate Ligands ")` : When a ligand can bind through two donor atoms as in `color{red}(H_2NCH_2CH_2NH_2)` (ethane-1,2-diamine) or `color{red}(C_2O_(4)^(2–))` (oxalate), the ligand is said to be didentate.
(iii) `color{green}("Polydentate Ligands ")` When several donor atoms are present in a single ligand as in `color{red}(N(CH_2CH_2NH_2)_3)`, the ligand is said
to be polydentate.
`color{red}("Example ")` : Ethylenediaminetetraacetate ion (`color{red}(EDTA^(4–))`) is an important hexadentate ligand. It can bind through two nitrogen and four oxygen atoms to a central metal ion.
(iv) `color{green}("Chelate Ligands ")` : When a di- or polydentate ligand uses its two or more donor atoms to bind a single metal ion, it is said to be a chelate ligand.
● Complexes formed by chelating ligands are called chelate complexes.
● The complexes tend to be more stable than similar complexes containing unidentate ligands.
(v) `color{green}("Ambidentate Ligands ")` Ligand which can ligate through two different atoms is called ambidentate ligand.
● Examples of such ligands are the `color{red}(NO_(2)^-)` and `color{red}(SCN^-)` ions.
`->` `color{red}(NO_(2)^-)` ion can coordinate either through nitrogen or through oxygen to a central metal atom/ion.
`->` `color{red}(SCN^-)` ion can coordinate through the sulphur or nitrogen atom.